Our group has measured, correlated, or modeled the thermophysical properties of seawater, ground water, and a range of produced waters. Our work has resulted in an open-source code for calculating seawater properties (available at: http://web.mit.edu.ezproxy.canberra.edu.au/seawater). We have made high-precision measurements of seawater surface tension over a broad range of temperature and salinity. We have compiled solubility data suitable for analyzing arbitrary produced waters. We have also collaborated with the US Geological Survey to analyze the composition of tens of thousands of ground water samples, and we have quantitatively evaluated the limitations of simplified activity coefficient models for important electrolytes.
Our seawater surface tension correlation has been adopted by the International Association for the Properties of Water and Steam (IAPWS) as an international standard guideline.
Selected Papers on Thermophysical Properties of Saline Water
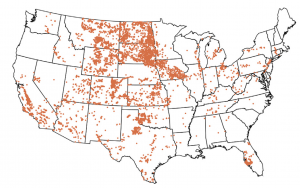
Groundwater samples for which sulfate is >50% of anion concentration (Ahdab et al., Water Research, 2018).
K.G. Nayar, M.H. Sharqawy, L.D. Banchik, and J.H. Lienhard V, “Thermophysical properties of seawater: A review and new correlations that include pressure dependence,” online 7 April 2016, Desalination, 390:1-24, 15 July 2016. (doi link) (preprint) Extends and updates our 2010 correlations for pressure variations and improved accuracy.
K.G. Nayar, D. Panchanathan, G.H. McKinley, and J.H. Lienhard V, “Surface tension of seawater,” J. Phys. Chem. Ref. Data, 43(4):43103, Nov. 2014. (doi link) (preprint)
New measurements and a reference correlation for seawater surface tension of seawater across a salinity range of 20 ⩽ S ⩽ 131 g/kg and a temperature range of 1 ⩽ t ⩽ 92 °C. IAPWS has adopted the correlation in this paper as an international standard guideline.
G.P. Thiel and J.H. Lienhard V, “Treating produced water from hydraulic fracturing: composition effects on scale formation and desalination system selection,” Desalination, 346:54-69, May 2014. (doi link) (preprint) Extensive use of the Pitzer-Kim model and tabulations of the standard-state Gibbs free energy of formation, the enthalpy of formation, and the molar specific heat capacity for aqueous and solid compounds.
K. Mistry, H.A. Hunter, and J.H. Lienhard V, “Effect of composition and nonideal solution behavior on desalination calculations for mixed electrolyte solutions with comparison to seawater,” Desalination, 318:34-47, June 2013. (doi link) Quantitatively evaluates the break-down of elementary coefficient models (e.g., Davies) as concentration rises and provides better alternatives.
K.H. Mistry and J.H. Lienhard V, “Effect of Nonideal Solution Behavior on Desalination of a Sodium Chloride (NaCl) Solution and Comparison to Seawater,” J. Energy Res. Tech., 135(4):042003, Dec. 2013. (doi link) Includes a correlation for the osmotic coefficient of NaCl(aq) for molality from zero to saturation.
M.H. Sharqawy, J.H. Lienhard V, and S.M. Zubair, “On Exergy Calculations for Seawater with Application to Desalination Systems,” International Journal of Thermal Sciences, 50(2):187-196, Feb. 2011. (doi link) (pdf)
M.H. Sharqawy, J.H. Lienhard V, and S.M. Zubair, “The thermophysical properties of seawater: A review of existing correlations and data,” Desalination and Water Treatment, 16:354-380, April 2010. (pdf)
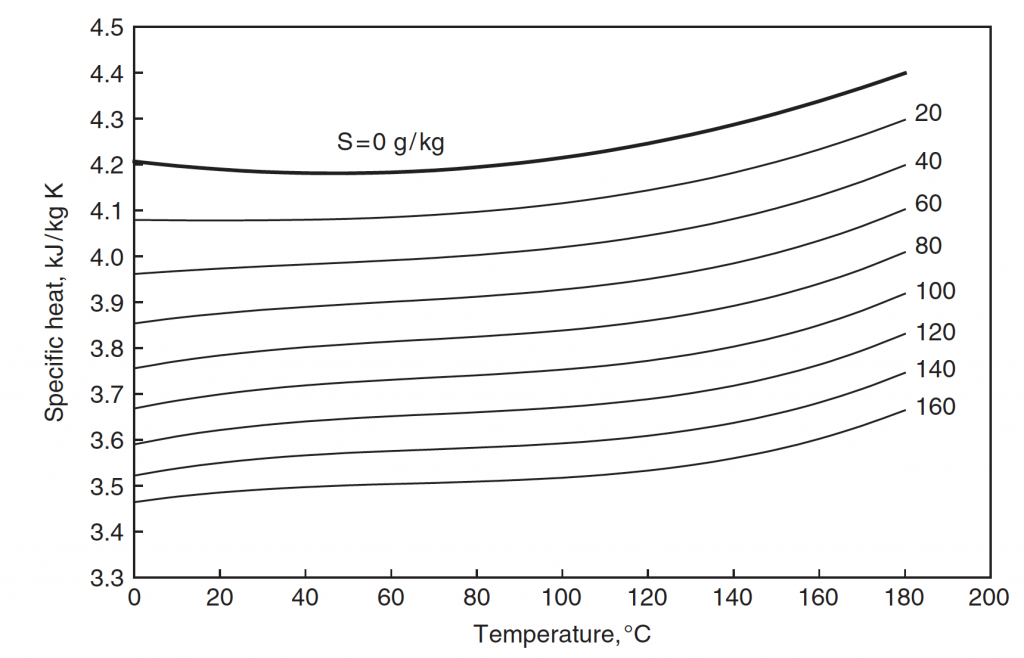
Isobaric specific heat capacity of seawater as a function of temperature and salinity (from Sharqawy et al., 2010)